Benign intracranial hypertension associated with inhaled corticosteroids in a child with asthma
- 1 Department of Paediatrics, Children's Health Ireland at Tallaght University Hospital, Dublin, Ireland
- 2 Department of Neurology, Children's Health Ireland at Temple Street, Dublin, Ireland
- 3 Department of Ophthalmology, Royal Victoria Eye and Ear Hospital Dublin, Dublin, Ireland
- 4 Faculty of Health Sciences, Trinity College Dublin, Dublin, Ireland
- Correspondence to Dr James Trayer; trayerj@tcd.ie
Abstract
A 13-year-old male asthmatic presented to the general paediatric clinic with papilloedema identified following a check-up with his optician due to blurred vision. His asthma was well controlled on a moderate dose of inhaled corticosteroid and there had been no recent increase or decrease in the dose. A diagnosis of benign intracranial hypertension (BIH) was made based on a raised cerebrospinal fluid opening pressure, papilloedema, a normal neurological examination and normal neuroimaging. The only associated risk factor was his inhaled corticosteroids. He was commenced on acetazolamide and the inhaled corticosteroid dose was reduced, resulting in resolution of his papilloedema. This case serves to highlight that steroid side effects including BIH may occur at moderate doses of inhaled corticosteroids and that inhaled corticosteroid dose should be regularly reviewed and decreased to the lowest dose that maintains asthma control.
Background
Benign intracranial hypertension (BIH), also known as idiopathic intracranial hypertension and pseudotumor cerebri syndrome, is a rare neurological condition characterised by raised intracranial pressure and papilloedema. Neuroimaging and cerebrospinal fluid (CSF) analysis are normal. It is most commonly associated with obesity and occurs more often in women than men. The typical presenting features are headache and visual disturbance. There may be associated cranial nerve palsies. Although described as a benign condition, there can be severe and permanent visual impairment if left untreated. While BIH is often idiopathic, it can be associated with medications such as retinoids, tertacyclines and corticosteroids. Corticosteroids can cause BIH when used for prolonged periods of time. Withdrawal of corticosteroids leading to adrenal insufficiency can also lead to the development of BIH.
This case involves a teenager with asthma who developed BIH associated with moderate-dose inhaled corticosteroids without evidence of adrenal insufficiency.
Case presentation
A 13-year-old boy with a history of asthma, eczema and allergic rhinitis was seen in the general paediatric clinic with papilloedema. He had presented to his optician with blurred vision who was concerned about the development of papilloedema that had not been present previously. There were no associated headaches, tinnitus, transient visual obscurations, behavioural changes or vomiting.
His asthma was well controlled since an admission 18 months previously on fluticasone propionate 125 µg two puffs twice a day (total dose 500 µg daily) and montelukast 5 mg at night. His mother reported excellent compliance with his medication and he rarely missed a dose. He remained asymptomatic with no exertional or nocturnal symptoms, nor required any oral steroids for over a year. Apart from his inhaled fluticasone, he was not taking any other forms of corticosteroids.
On examination, he had a normal neurological and respiratory examination. He was growing along the 90th centile for weight and between 75th and 90th centile for height with a normal BMI of 21 kg/m2. He was reviewed by a consultant ophthalmologist who confirmed bilateral papilloedema on funduscopy and orbital ultrasound scan (see figures 1 and 2). The history and examination findings were compatible with raised intracranial pressure and he was referred for a lumbar puncture to assess CSF opening pressure.
Left eye funduscopic appearance demonstrating papilloedema.
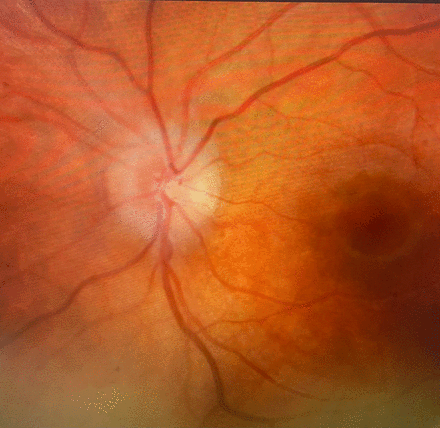
Right eye funduscopic appearance demonstrating papilloedema.

Investigations
An MRI brain scan was performed which demonstrated an incidental finding of an arachnoid cyst in the middle cranial fossa on the left side. There was no mass effect or oedema and no significant abnormality of the orbits. Lumbar puncture was performed which demonstrated an elevated opening pressure of 30 cm of H2O. CSF microscopy and biochemical analysis was normal. Investigations for potential causes of raised intracranial pressure including vitamins A and D, autoimmune disease, anti-MOG antibodies and thyroid function tests were all normal or negative. In addition, a cortisol level checked at 9:00 was 546 nmol/L making adrenal suppression very unlikely.
Differential diagnosis
A diagnosis of raised intracranial pressure was made based on the high CSF opening pressure. A diagnosis of BIH was made based on the American Academy of Neurology diagnostic criteria.1
BIH is most often seen in patients with obesity or significant recent weight gain, and it is also more common in females. While this patient had gained 15 kg in the preceding year, his weight remained in proportion to his height with a normal BMI.
Vascular causes of BIH were excluded based on normal MRI. Hypervitaminosis A can also cause BIH, but the level was normal in this case. Adrenal suppression secondary to medications or autoimmune disease has been associated with BIH, but this was excluded based on a normal early morning cortisol level. High-dose corticosteroids have been associated with the development of BIH. This is most often seen in patients on long-term oral corticosteroids or due to adrenal insufficiency following withdrawal of treatment.
In this case, a diagnosis of BIH associated with inhaled corticosteroids was made based on the aforementione findings.
Treatment
The patient was referred to a paediatric respiratory consultant for ongoing care of his asthma. The dose of inhaled corticosteroid was slowly weaned over the following 3 months. His asthma is currently well controlled on Seretide (fluticasone propionate 50 µg and salmeterol 25 µg) one puff twice a day (total dose 100 µg daily). He was commenced on oral acetazolamide and this has been weaned with an aim to discontinue in the coming months.
Outcome and follow-up
The patient’s papilloedema improved following reduction in the dose of corticosteroids. At no point did he develop a headache or vomiting.
Follow-up ophthalmology assessment demonstrated improvement in the appearance of the papilloedema and is now fully resolved 3 months after presentation with retinal nerve fibre layer thickness within normal limits. Visual field examination was normal and visual acuity was at his pre-morbid baseline 6 months following initial presentation.
Discussion
Corticosteroids have been implicated in cases of BIH when used at high doses for prolonged periods of time and secondary to adrenal insufficiency following discontinuation of corticosteroids. The mechanism underlying this is not fully understood, but it has been hypothesised that corticosteroid metabolism plays a role in CSF volume regulation as the enzyme 11β hydroxysteroid dehydrogenase is present in the choroid plexus.2
There are historic cases of high-dose corticosteroids leading to BIH reported in the literature.3–5 These patients were on chronic oral corticosteroids for the treatment of conditions such as systemic lupus erythematosus and inflammatory bowel disease as well as asthmatic children or oral steroids prior to the development of inhaled corticosteroids. More recently, Bond et al reported a case of a 13-year-old boy who developed a sixth cranial nerve palsy, headaches and papilloedema while taking fluticasone propionate nasal spray (50 µg one spray to each nostril daily) for the treatment of allergic rhinitis.6 While CSF opening pressure was not measured, a presumed diagnosis of BIH was made. Following discontinuation of the nasal corticosteroid, the patient’s symptoms resolved.
BIH is more commonly related to adrenal insufficiency secondary to prolonged corticosteroid use. In these cases, the symptoms develop shortly after discontinuation of the corticosteroid. This has been reported in children with eczema treated with high potency topical steroids as well as asthmatics on reducing doses of oral steroids.7–9 More recently, there have been reports of asthmatic children developing BIH following reduction in the dose of their inhaled corticosteroids.10–12 Studies have demonstrated that adrenal insufficiency is common in patients on inhaled corticosteroids and is related to longer duration of treatment and the use of higher doses.13 This case differs from the two mentioned previously as there was no recent decrease in the dose of inhaled corticosteroids our patient was taking. There had also been no recent increase in the dose or addition of other sources of steroids. A normal early morning cortisol level also suggests that there was no significant adrenal suppression. To the best of our knowledge, this is the first reported case of BIH secondary to inhaled corticosteroids unrelated to adrenal insufficiency following a reduction in the dose. This is despite the fact that the dose of corticosteroid that was prescribed is considered within the moderate range for the patient’s age based on international guidelines.14 15 Inhaled corticosteroids are effective in the prevention of exacerbations of asthma and are generally well tolerated. Significant side effects are uncommon but are seen more frequently at higher daily doses.16 This case serves to remind us that asthmatics whose symptoms are well controlled should have their medications regularly reviewed and should have their treatment stepped down to the lowest dose that maintains good symptom control.
Patient’s perspective
This has been a scary and difficult experience for us all but we feel that we have got excellent care for our child’s asthma and for the BIH. We understand that he was on a moderate dose of inhaled corticosteroid and have learned that some children may experience side effects at this dose.
We still believe the medication is very important to keep his asthma controlled and he is currently taking a lower dose inhaled corticosteroid combined with another preventer medication. This seems to work even better than the previous medication.
The care we got during this time has also been excellent due to very professional medical staff. While it has not changed our experience with asthma medication, we will be more cautious with medications in the future and aware of potential side effects.
Learning points
-
Benign intracranial hypertension is a side effect of chronic corticosteroid use and may present in children on long-term corticosteroids or due to the resulting adrenal insufficiency following their discontinuation.
-
Benign intracranial hypertension is diagnosed based on a raised cerebrospinal fluid (CSF) opening pressure (>28 cm H2O) with normal CSF composition, papilloedema, normal neuroimaging and a normal neurological examination.
-
While generally well tolerated, moderate-dose inhaled corticosteroids can lead to the development of steroid-related side effects including benign intracranial hypertension.
-
Patients with well-controlled asthma should regularly have their medications reviewed to ensure control is maintained at the lowest possible dose.
Acknowledgments
We would like to thank the patient and his parents for their assistance in writing this report.
Footnotes
-
Contributors JT and BE were involved in planning the paper. JT, DOR and LC reviewed the clinical case notes. All authors were involved in drafting and reviewing the manuscript. All authors have given their approval for the final draft.
-
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
-
Competing interests None declared.
-
Provenance and peer review Not commissioned; externally peer reviewed.
- © BMJ Publishing Group Limited 2021. No commercial re-use. See rights and permissions. Published by BMJ.
References
Use of this content is subject to our disclaimer